

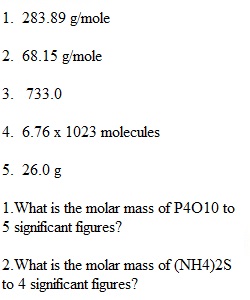
Q 1.What is the molar mass of P4O10 to 5 significant figures? 2.What is the molar mass of (NH4)2S to 4 significant figures? 3.How many grams of biotin (C10H16N2O3S) are in 3 moles? 4.How many molecules are in 137g of the B vitamin Nicotinamide. (C6H6N2O)? 5.How many grams of glucose (C6H12O6) are consumed if 15.6 ml of water are produced? (density = 1.00 g/ml) C6H12O6 + 6O2 -> 6H2O + 6CO2
View Related Questions